![]()
Prevention and treatment of dry syndromes and obesity, application to regenerative medicine
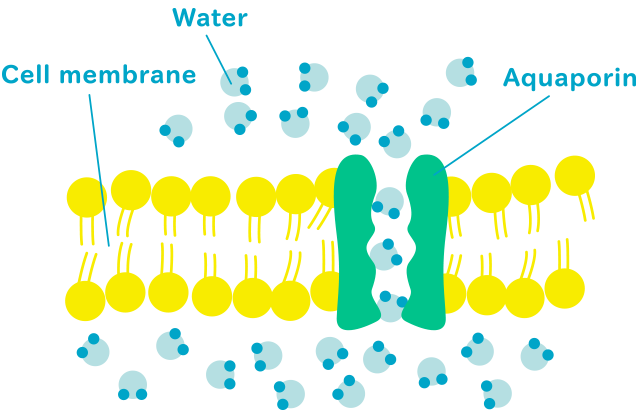
The human body is 70% water approximately. Water in the body includes intracellular water, and extracellular water (e.g. plasma, interstitial, and transcellular fluid). The fluid never stops and yet the water never stays the same, circulating through the cells and whole-body. The key regulator of the water circulation is the aquaporin* protein. Aquaporins are expressed in the cell membrane and selectively permeable to water for water transport to the body.
In all, 13 aquaporins have been identified in humans. A unique set of aquaporins is expressed in each tissue, and each aquaporin has unique characteristics and performs unique functions. We will focus on aquaporins 3 and 7 (AQP3 and AQP7, respectively) in the present research. By examining the functions of these aquaporins, we aim to establish a method for modulating water and lipid metabolism at the cellular and whole-body level to improve human health and longevity.
- *
Aquaporins were discovered by Dr. Peter Agre (winner of the Nobel Prize in Chemistry in 2003) of the United States in 1992.
Aquaporins are expressed in the cell membrane and act as water channels. AQP3, AQP7, AQP9, and AQP10 are called aquaglyceroporins because they are permeable to glycerol, urea, and other small solutes, as well as water. Aquaglyceroporins function as the regulators of lipid and water circulation across the body.
Current research projects are as follows:
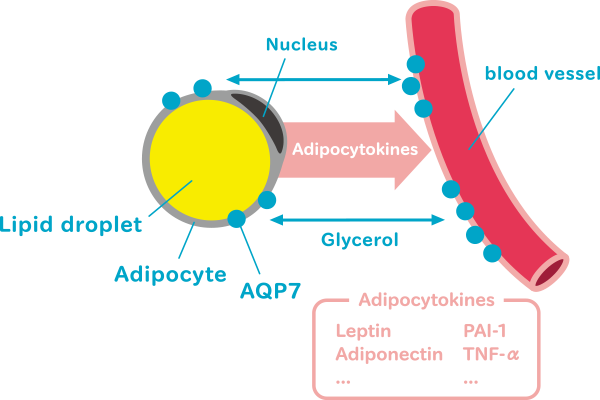
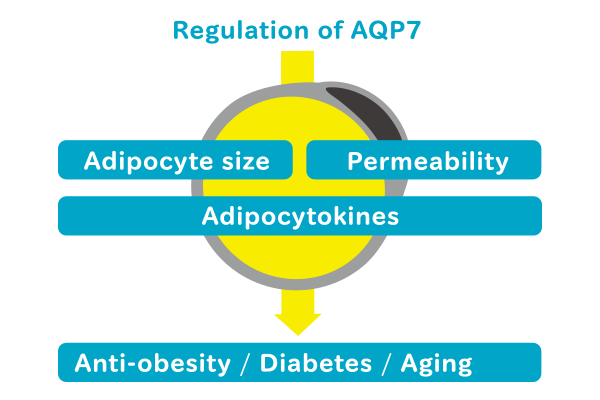
AQP7 is a putative multifunctional protein. AQP7 function of interest to us is connecting degradation and accumulation of lipids. We wish to identify molecular mechanism underlying and upstream and downstream participants involved in the signal transduction pathway promoting the degradation and accumulation of lipids. We also wish determine the metabolic role of AQP7 expressed in the blood vessels of adipose tissue.
Adipocytes in the white adipose tissue maintain the homeostasis of whole-body energy by synthesizing and accumulating and by degrading and releasing lipids. Adipocytes also produce and release many bioactive factors called adipocytokines (e.g., leptin, adiponectin, TNF-alpha, and PAI-1) into the blood, so appropriate regulation of adipocytes can aid in the appropriate regulation of whole-body metabolism. Moreover, appropriate regulation of stored lipids involves adjusting the balance between the synthesis and degradation of these lipids, disruption of this balance therefore results in metabolic disorders (such as type 2 diabetes). However, mechanisms underlying the maintenance of this balance are unclear.
In this research project, we aim to determine mechanisms underlying systemic metabolic regulation in the white adipose tissue by analyzing AQP7 signaling, to positively modulate lipid metabolism and human health.
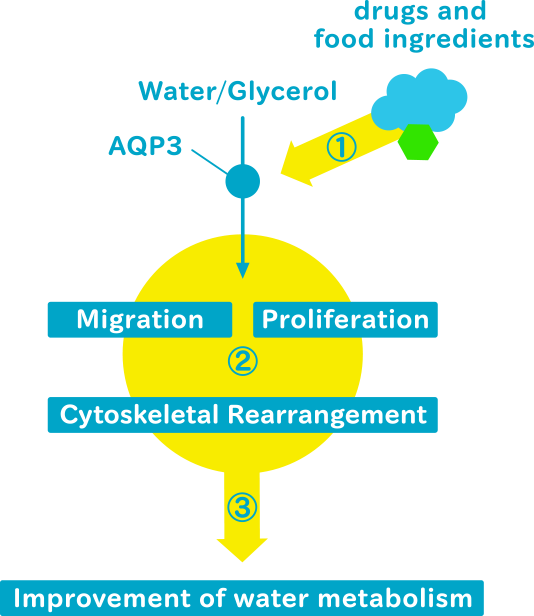
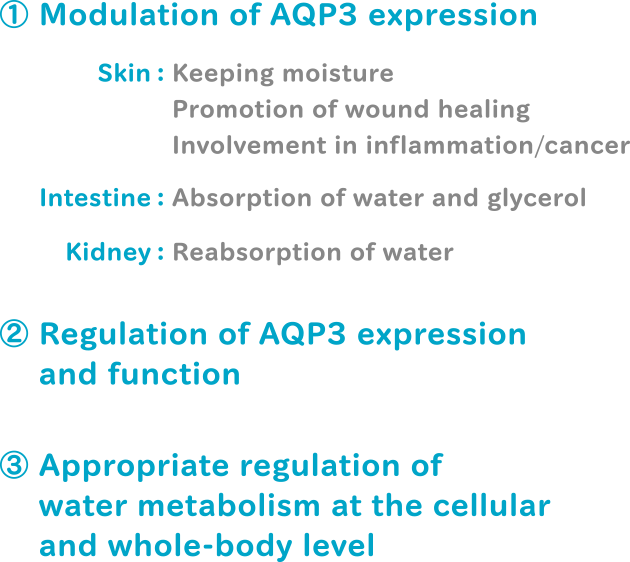
We intend to determine a method for regulating AQP3 by using external stimuli such as drugs or foods, connecting to positively modify skin condition. We also aim to determine how AQP3 alters the barrier function of the skin. Regulation of AQP3 expression and function may improve the function/texture of the epithelium and promote skin health.
AQP3 plays an important role in skin hydration and cell proliferation, in addition to urine-concentration. Aqp3-deficient mice show poor skin hydration and elasticity, delayed wound healing, and impaired retinoic acid-induced keratinocyte proliferation.
We will particularly focus on AQP3 in the skin. Modulation of AQP3 expression can counteract the decrease in the sustainability of skin structure due to dermatitis or aging. This approach is promising for maintaining the structure of the skin and for treating epithelial disorders. Restoration of age-related decrease in AQP3 expression may find application in the esthetic field such as rejuvenation of skin texture. Furthermore, we intend to elucidate mechanisms underlying AQP3-associated intracellular signaling.